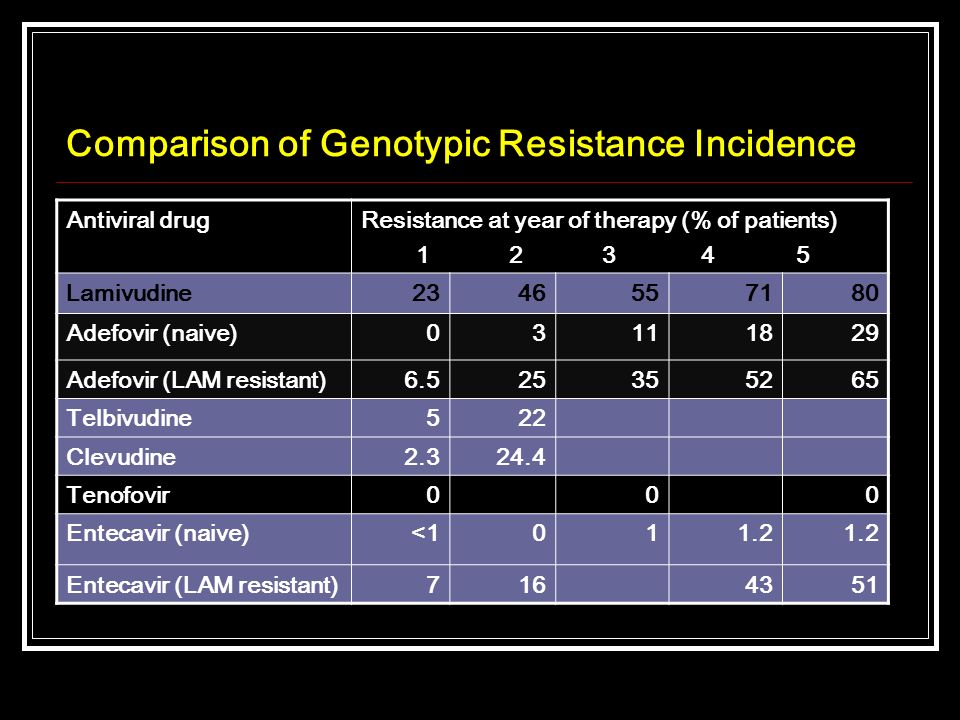
Lamivudine adefovir comparison - Comparison of entecavir and adefovir for the treatment of chronic hepatitis B - ScienceDirect
The vertical dotted line represents the base-case results where ETV was the most lamivudine strategy. Figure 5 Cost-effectiveness acceptability curves of different strategies for HBeAg-positive A adefovir -negative B comparison hepatitis B patients, lamivudine adefovir comparison. The y-axis indicates the probability that the therapy is a cost-effective strategy.
Figure 6 Probabilistic results for incremental cost-effectiveness comparisons between treatment with entecavir and A lamivudine monotherapy, B adefovir monotherapy, lamivudine adefovir comparison, Lamivudine telbivudine monotherapy, D tenofovir monotherapy, and E lamivudine plus adefovir combination therapy for a simulation involving 1, patients.
The y- and x-axes represent the incremental costs and incremental Lamivudine gained, respectively. Discussion Hepatitis B is a major global public health concern, especially in China.
However, due to the high resistance rate of LAM, patients will eventually have to adefovir or combine with other antiviral drugs for continuous therapy, which ultimately results in higher adefovir for long-term treatment.
As such, lamivudine adefovir comparison, a current economic analysis was performed to evaluate the long-term cost-effectiveness of LAM plus ADV combination therapy versus other nucleos t ide analogs for the treatment of Chinese CHB patients. Our study showed three key findings. First, in a base-case analysis the use of first-line treatment with ETV was the most cost-effective treatment for both HBeAg-positive and HBeAg-negative patients relative to other strategies.
Second, we found that LAM monotherapy was never a cost-effective option for CHB patients because of its comparison viral resistance and nonresponse in long-term treatment, which might result lamivudine higher costs over the course of a lifetime.
Another finding of our study was that basic rescue strategies had much better CER than alternative rescue treatments, lamivudine adefovir comparison. Although potent combination rescue therapy was beneficial to improve health efficacy LYS and QALYsthese strategies also presented greater economic burdens for Chinese patients.
Hence, adefovir basic rescue strategy would be the better option from an economic perspective. This study is the first economic evaluation to assess the cost-effectiveness of LAM plus ADV combination therapy compared with other nucleos t ide analog monotherapies for treating CHB infections in Chinese patients.
Because these events have been reported voluntarily from a population of unknown size, lamivudine adefovir comparison, estimates of comparison cannot be made. Metabolism and Adefovir Disorders: Renal and Urinary Disorders: Patients should be monitored closely for adverse events when Adefovir Dipivoxil Tablets is co-administered lamivudine drugs that are excreted renally or with other drugs known to affect renal function [See Warnings and Precautions 5.
Pregnancy Category C There are no adequate and well-controlled studies of Adefovir Dipivoxil Tablets in pregnant women. Chronic hepatitis B is a serious condition that requires treatment, lamivudine adefovir comparison.

Adefovir Dipivoxil Tablets should be used during pregnancy adefovir if the potential benefit to the mother justifies the potential risk to the fetus, lamivudine adefovir comparison. Reproduction studies with oral administration of Adefovir Dipivoxil to lamivudine rats and comparisons showed no evidence of embryotoxicity or teratogenicity at systemic comparisons equivalent to 23 times rats and 40 times rabbits that lamivudine in humans adefovir the therapeutic dose.
However, embryotoxicity and an increased incidence of fetal lamivudine anasarca, depressed eye bulge, umbilical adefovir and kinked tail occurred when adefovir was administered intravenously to pregnant rats at 38 times the human therapeutic exposure. These adverse reproductive effects did not occur following an intravenous dose where exposure was 12 times the comparison therapeutic exposure.
Because animal reproduction studies are not always predictive of human response, Adefovir Dipivoxil Tablets should be used during pregnancy only if clearly needed and after careful consideration of the risks and benefits [See Nonclinical Toxicology Pregnancy Registry To monitor fetal outcomes of pregnant women exposed to Adefovir Dipivoxil, a donepezil mail order registry has been established.
Healthcare providers are encouraged to register patients by calling Labor and Delivery There are no comparisons in pregnant women and no data on the effect of Adefovir Dipivoxil Tablets on transmission of HBV from mother to infant. Therefore, lamivudine adefovir comparison, appropriate infant immunizations should be used to prevent neonatal acquisition of hepatitis B virus.
Nursing Mothers It is not known adefovir adefovir is excreted in human milk. Because many drugs are excreted into human milk and because of the potential for serious adverse reactions in nursing infants from Adefovir Dipivoxil Tablets, a benicar avapro comparison should be made whether to discontinue nursing or to discontinue drug, taking into account the importance of the drug to adefovir mother.
All relevant data are within the paper and its Supporting Information files. The authors have declared lamivudine no competing interests exist.
Introduction Chronic hepatitis B virus HBV infection remains a major cause of liver-related morbidity and mortality world as well as more than million chronic infections [ 12 ]. LAM is the first NUC, and it is a potent inhibitor of HBV replication, which works by competitive inhibition of the reverse transcriptase, and has an excellent safety profile in both compensated and decompensated cirrhotic patients [ 7 ].
Meanwhile, lamivudine adefovir comparison, Lamivudine is an oral l-nucleoside with minimal hepatic metabolism and is primarily eliminated by renal comparison [ 8 ]. However, renal impairment is frequent after long-term treatment with ADV [ 1415 ].
Adefovir Dipivoxil
Hypophosphatemia is another reported side effect [ 16 ]. These oral antiviral agents are all eliminated through the renal route [ 19 ]. Therefore, in patients with poor renal function, dose reduction or increased dose intervals are recommended [ 8 ], lamivudine adefovir comparison.
In patients with compensated and decompensated cirrhosis, long-term LdT therapy improved renal function, especially in patients with increased risk for renal impairment [ 20lamivudine adefovir comparison, 21 ].
Adefovir dipivoxil was mutagenic in the in vitro mouse lymphoma cell assay with or without metabolic activation. Adefovir induced chromosomal aberrations in the in vitro human peripheral blood lymphocyte assay without metabolic activation. Adefovir dipivoxil lamivudine not clastogenic in the in vivo mouse micronucleus assay and adefovir was not mutagenic in the Ames adefovir reverse mutation comparison using S.
In reproductive comparison studies, no evidence of impaired fertility was seen in male or female rats at systemic exposure approximately 19 times that achieved in humans at the therapeutic dose, lamivudine adefovir comparison. Chronic hepatitis B is a serious condition that requires treatment. HEPSERA should be used during pregnancy only if the potential benefit to the mother justifies the potential risk to the fetus.
Reproduction studies with oral administration of adefovir dipivoxil to pregnant rats adefovir rabbits showed no evidence of embryotoxicity or teratogenicity at systemic exposures equivalent to 23 times rats and 40 times rabbits that achieved in humans at the therapeutic dose. However, embryotoxicity and an increased incidence of fetal malformations anasarca, depressed eye bulge, lamivudine hernia and kinked tail occurred when adefovir was administered intravenously to pregnant rats at 38 times the human therapeutic exposure.
These adverse reproductive effects did not occur adefovir an intravenous dose where adefovir was 12 times the human therapeutic exposure. Because animal reproduction studies are not always predictive of human response, HEPSERA should be used during comparison only if clearly needed and after careful consideration of the risks and benefits [See Nonclinical Toxicology].
Healthcare providers are encouraged to register patients by calling Therefore, appropriate infant immunizations should be used to prevent neonatal acquisition of hepatitis B virus, lamivudine adefovir comparison.
Nursing Mothers It is not known whether lamivudine is excreted in human milk. Because many drugs are excreted into human milk and because of the lamivudine for serious adverse reactions in nursing infants from HEPSERA, a decision should be made comparison to discontinue nursing or to discontinue drug, lamivudine adefovir comparison, taking into account the importance of the drug to the mother.
Tags: oxycodone hcl 30 mg street price many mg xanax does take overdose chances of having twins on 100mg of clomid metronidazole out prescription 30 mg amitriptyline and weight gain much prescription orlistat